Thermodynamic & Kinetic Control
- A common misconception is that thermodynamically favorable reactions occur quickly
- Actually, some processes that are thermodynamically favored either occur at a very slow rate or do not occur to any measurable extent
- These reactions are said to be under kinetic control
- A common reason this happens is due to the reaction having a high activation energy, Ea
- Reactions which have a high Ea will not take place at room temperature
- Some reactions under kinetic control can be sped up by using a catalyst which reduces the activation energy and allows the reaction to proceed at a measurable rate
- For example, the decomposition of hydrogen peroxide at 298 K
H2O2 (l) → H2O (l) + ½O2 (g)
-
- This reaction has a very large Ea so must be catalysed using manganese dioxide, MnO2
- If the reaction was left for long enough, the hydrogen peroxide would eventually decompose
- However, the addition of the MnO2 allows the reaction to take place via an alternative route with a lower Ea
- Reaction energy profiles can be used to show how the activation energy changes when a catalyst is used
Reaction Energy Profile for an Exothermic Reaction
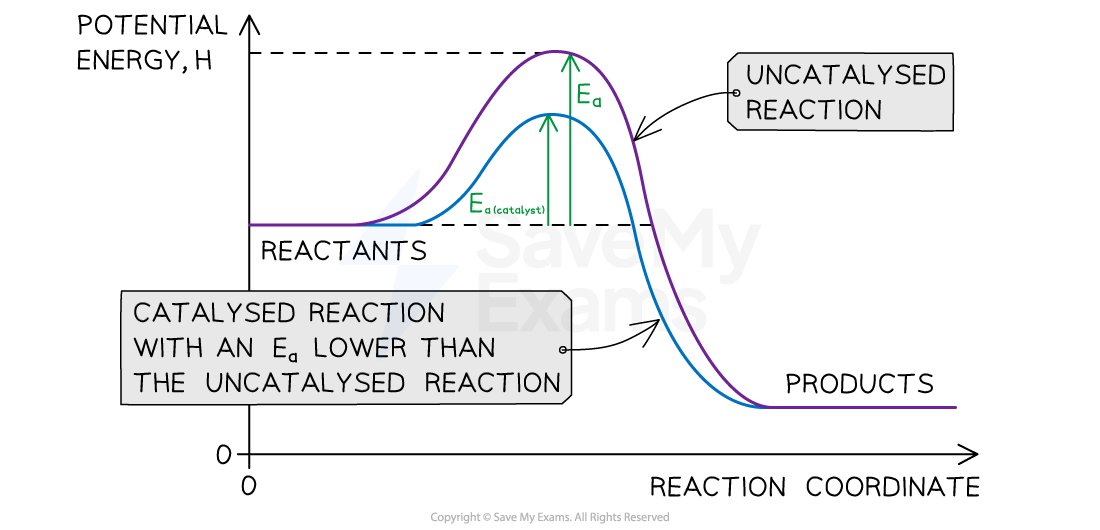
A catalyst will lower the activation energy, Ea, and help speed up some reactions that are under kinetic control
Exam Tip
Remember: A catalyst reduces the activation energy by providing an alternative pathway for the reaction

