Vapor Pressure & Boiling Point
- Intermolecular forces are broken or formed when solids and liquids change state
- The type and strength of the intermolecular forces can affect the observable properties of solids and liquids, including:
- Melting point
- Boiling point
- Vapor pressure
- Solubility
- Viscosity
- Surface tension
- Vaporization is when a liquid changes its physical state into a gas
- The reverse of this process, converting a gas back to a liquid, is known as condensation
What is vapor pressure?
- Liquids, and even some solids, are continuously vaporizing.
- If we place a quantity of ethanol (CH3CH2OH) in an evacuated, closed container, it quickly begins to vaporize
- This results in the development of an increasing pressure exerted by the vapor in the space above the liquid
- After a short time, the pressure of the vapor attains a constant value.
- This constant value is known as vapor pressure
Vapor Pressure
A vapor pressure develops in the evacuated closed container as an equilibrium is established between the rate of vaporization of liquid ethanol and the rate of condensation of gaseous ethanol.
The vapor pressure of liquids
- The vapor pressure of a liquid is the pressure exerted by its vapor when the liquid and vapor are in dynamic equilibrium
- A dynamic equilibrium describes a state in which the rate of vaporization and condensation of a liquid are equal
- Volatile substances have high vapor pressures
- This means that they evaporate quicker than substances with low vapor pressure
- Generally, vapor pressure increases with temperature
- This is because more molecules of the liquid have sufficient energy to break the intermolecular forces and change to vapor
- The vapor pressure of a liquid depends on intermolecular forces
- A liquid with stronger intermolecular forces has a lower vapor pressure because there is a greater attraction between the molecules
What is the Boiling Point?
- The temperature at which the vapor pressure of a liquid equals the pressure exerted on the liquid (atmospheric pressure, unless the vessel containing the liquid is closed) is called the boiling point of the liquid
- The temperature at which a given liquid boils increases with increasing external pressure.
- When the pressure is 1 atm, the boiling point is known as normal boiling point
- At a given external pressure, liquids with low vapor pressures have a higher boiling point while volatile liquids have low boiling points
- So, stronger intermolecular forces mean a lower vapor pressure AND a higher boiling point
Vapor Pressure Vs Temperature Graph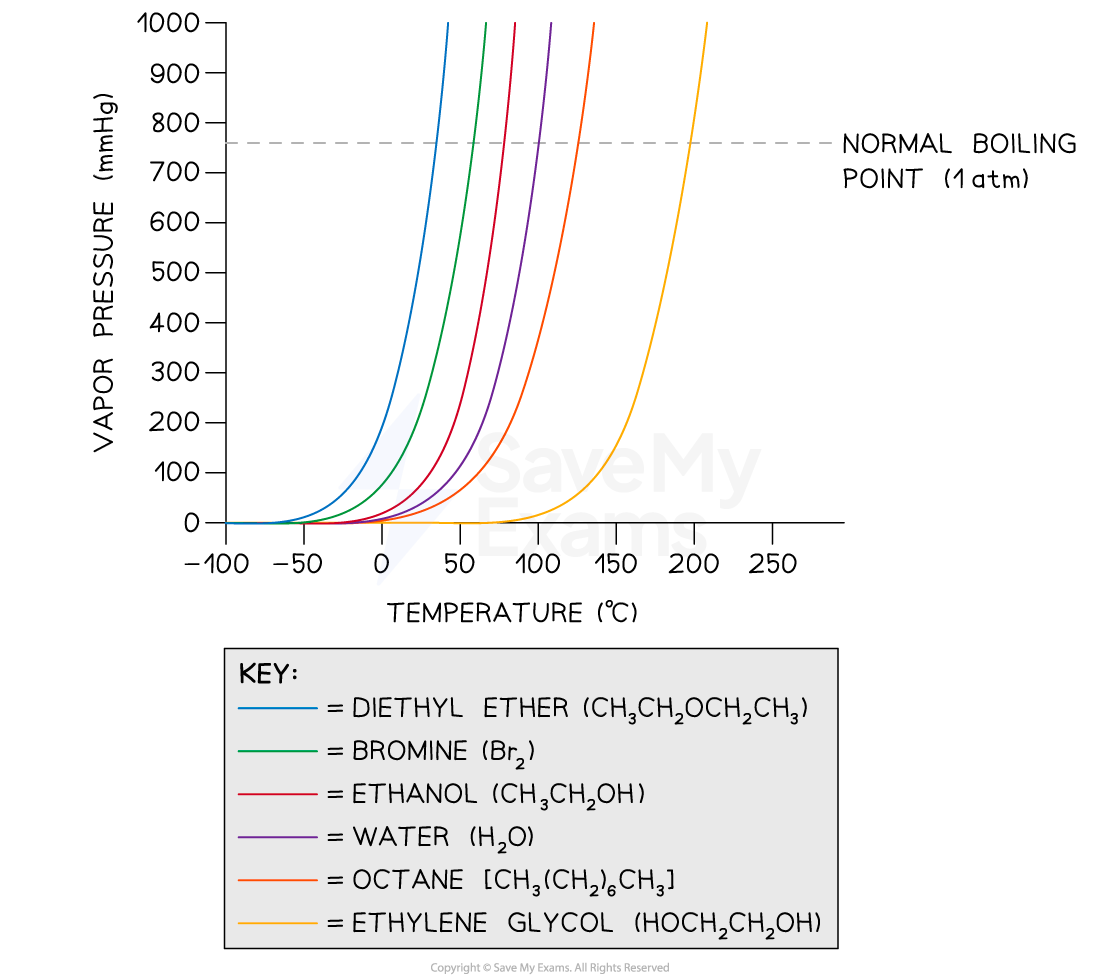
A graph showing the relationship between vapor pressure of several covalent compounds and their boiling points



