Molecular Collisions
- On a molecular level, the effect of temperature and concentration can be better understood by the collision model
- The key idea of the collision model is that molecules must collide to react
- Basically, the greater the number of collisions between molecules per second, the faster the reaction
- Hence, when the concentration of reactants increases, the number of collisions increases resulting in an increased rate of reaction
- According to the kinetic-molecular theory, increasing the temperature increases the average kinetic energy of the molecules
- This makes the molecules move faster, colliding more frequently and with more energy which then means a faster reaction rate
- An increase in the surface area of solid reactants also increases the reaction rate
- As a result of the increase in the number of contact points for collision and the consequent increase in the frequency of collision
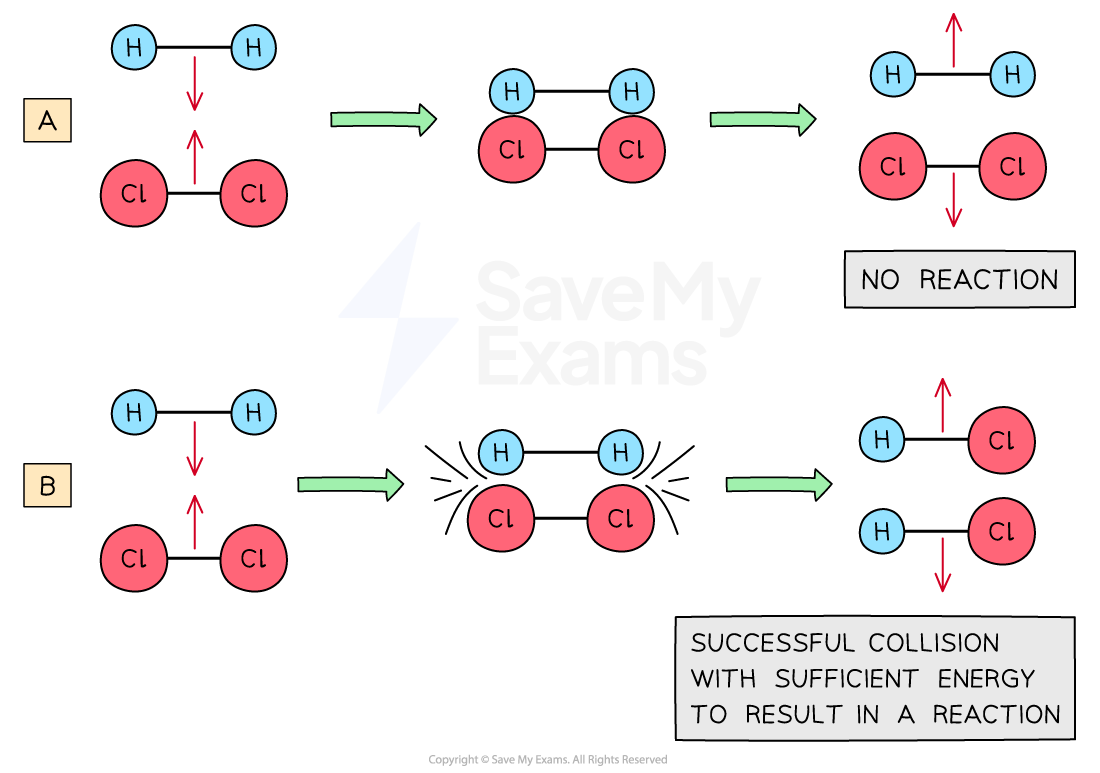
- However, it is important to note that not every collision between reactant molecules results in product formation
- Collisions which result in reactions are said to be effective collisions and possess two characteristics:
- Reacting molecules must collide with sufficient energy greater or equal to some minimum energy known as the activation energy (kinetic energy factor)
- Reacting molecules are properly oriented towards each other (orientation factor)
Orientation Factor
- The relative orientations of the molecules during collision determine whether the atoms are suitably positioned to form new bonds
- Essentially, molecules have to be in the correct position to react
- Consider the reaction between CO and NO2 to form CO2 and NO:
- The reaction only happens when the carbon atom of the CO molecule strikes an oxygen atom of the NO2 molecule
Orientation Factor and Effective Collision

Diagram showing the effect of proper orientation of colliding molecules for a successful reaction. In the reaction between CO and NO2, a successful collision is obtained when the carbon atom of the CO strikes the oxygen atom of the NO2.
- According to the collision model, the rate constant, k, which indicates the rate of a reaction, is a product of three factors:
- Z, the collision frequency, which gives the number of molecular collisions occurring in unit time at unit concentrations of reactants
- f, the fraction of collisions having energy greater than the activation energy
- p, the steric factor, which accounts for the fraction of collisions that occur with the reactant molecules properly oriented
- With this, the collision model can be made quantitative with the expression:
k = p × Z × f
Worked example
Based on the collision model, which of the following statements explains the energy requirements of a reaction?
- The rate of reaction increases with both increasing temperature and activation energy
- The rate of reaction increases with both decreasing temperature and activation energy
- The rate of reaction increases with both increasing temperature and decreasing activation energy
- The rate of reaction increases with both decreasing temperature and increasing activation energy
Answer:
Option C is correct because:
- An increase in temperature increases the average kinetic energy of the reacting particles leading to a faster reaction
- A decrease in activation energy increases the fraction of molecules with effective collisions leading to a faster reaction