The Structure of Solids
- Solids are a nearly incompressible state of matter with well defined shapes
- This is because they are made of atoms, molecules and ions which are in close contact and in fixed positions
- These particles do not move relative to each other
- This explains why solids do not flow, unlike liquids
- We can visualize a solid as being formed by stacking a large number of small, identical structural units, in the same manner as building a wall by stacking identical bricks
- Structurally, solids can be divided into two categories:
- Crystalline
- Amorphous
- Solids in which atoms are arranged in an orderly repeating pattern are called crystalline solids
- The arrangement of particles in a crystalline solid is such that the net attractive intermolecular forces are at their maximum
- The forces responsible for the stability of a crystal can be
- Ionic forces
- Covalent bonds
- Van der Waals forces
- Hydrogen bonds
- Or a combination of these forces
- When most liquids are cooled, they eventually freeze and form crystalline solids
- Examples of crystalline solids include sodium chloride, sucrose, ice and diamond
- An amorphous solid has a disordered structure; it lacks the well-defined arrangement of basic units found in a crystal
- The structures of amorphous solids are similar to those of liquids on an atomic level, but the molecules, atoms and / or ions lack the freedom of motion they have in liquids
- They may be formed when liquids cool too quickly before their molecules become arranged in an orderly pattern
- Common examples of amorphous solids are rubber, glass and obsidian
Structures of crystalline and amorphous solids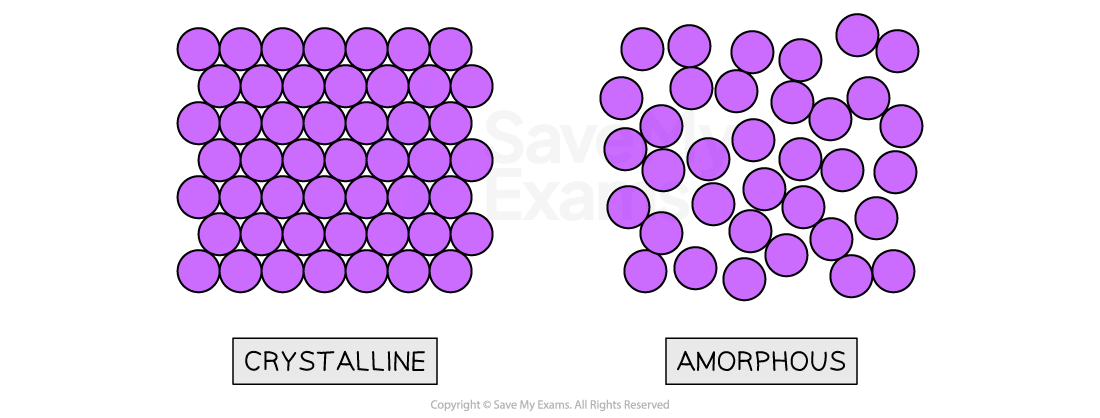
The particles in solids may be arranged in a regular repeating pattern (crystalline) or randomly (amorphous)

