Evaporation & Condensation (Oxford AQA IGCSE Physics)
Revision Note

Author
Ann HowellExpertise
Physics Content Creator
Evaporation & Condensation
The cooling effect of evaporation
Energy can be transferred by evaporation
Evaporation is a change in the state of a liquid to a gas
It has a cooling effect
Evaporation is different to boiling or vaporisation
Evaporation happens at any temperature, whereas boiling only occurs at the boiling point of the substance
Evaporation only occurs to particles at the surface of the liquid, whereas every particle changes state when a liquid is vaporised
The molecules in a liquid have a range of energies
Some have lots of kinetic energy, others have very little
Their average kinetic energy relates to the temperature of the liquid
Evaporation occurs when more energetic molecules moving near the surface of the liquid have enough energy to overcome the intermolecular forces of attraction holding the molecules in their liquid state
The average energy of the liquid is reduced
Therefore liquids are cooled down by evaporation
Evaporation from the surface of a liquid
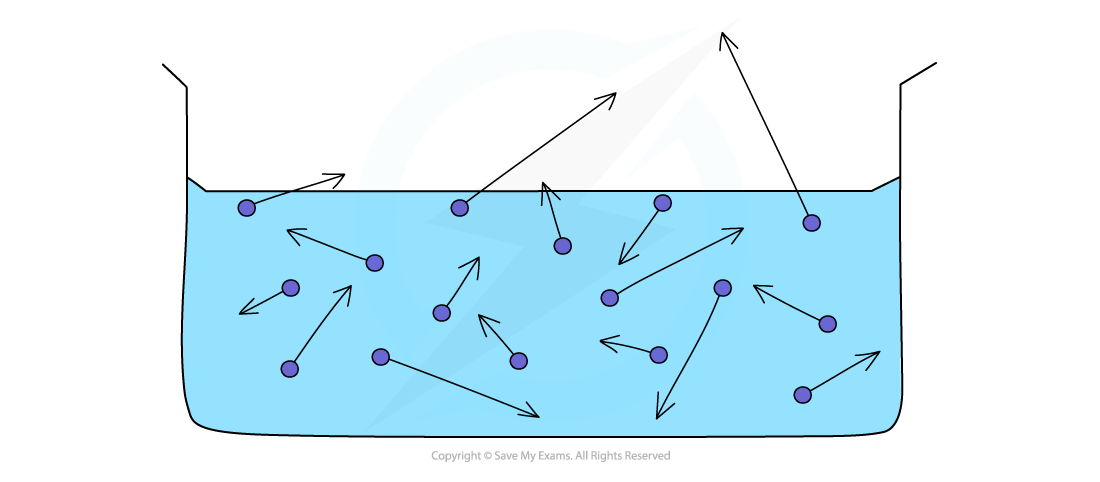
The process of evaporation can be used to cool things down:
If an object is in contact with an evaporating liquid, as the liquid cools the solid will cool as well
This process is used in refrigerators and air-conditioning units
Factors affecting the rate of evaporation
Increased temperature increases the kinetic energy of the molecules in the liquid
Molecules with more energy are more likely to overcome the intermolecular forces holding them in their liquid state and escape the surface
Therefore higher temperatures lead to a higher rate of evaporation
Molecules only escape the intermolecular forces of attraction at the surface of the liquid
Therefore a larger surface area leads to a higher rate of evaporation
Air movement carries away the water vapour which has just evaporated
This dries the air and allows more water molecules to escape
Therefore increasing air movement (when indoors this is sometimes called draughts) increases the rate of evaporation

You've read 0 of your 0 free revision notes
Get unlimited access
to absolutely everything:
- Downloadable PDFs
- Unlimited Revision Notes
- Topic Questions
- Past Papers
- Model Answers
- Videos (Maths and Science)
Did this page help you?