Practical: Investigating Diffusion & Osmosis (Edexcel IGCSE Biology: Double Science)
Revision Note

Author
LáraExpertise
Biology Lead
Practical: Factors that Influence Diffusion
- Diffusion is the movement of molecules from a region of higher concentration to a region of lower concentration
- The rate of diffusion is influenced by several factors:
- Temperature
- Surface area
- Concentration gradient
- Diffusion distance
- You can investigate how temperature affects diffusion using beetroot
- Beetroot cells contain a dark purple-red pigment
- Heating above 45℃ can damage the cell membrane meaning that the pigment can leak out
- The speed at which this pigment leaks out of the cell tells us about the rate of diffusion
Investigating the effect of temperature on diffusion
Apparatus
- Beetroot
- Knife
- Cork borer (optional)
- Cutting board
- Ruler
- Test tubes
- Water baths
- Stopwatch
Method
- Using a knife, cut 2 equally-sized cubes of beetroot
- The pieces must have the same dimensions so that they all have equal surface areas and volumes, as these factors could affect the rate at which the pigment leaks out
- A cork borer can also be used, as long as the cores are cut to the same length
- Rinse the beetroot pieces
- To remove any pigment released during cutting
- Put 5 cm3 of water into 2 test tubes labelled A and B
- Keep test tube A at room temperature and transfer test tube B to a hot water bath at 90℃
- Leave the test tubes for 2 minutes, then add a piece of beetroot into each test tube
- After 10 minutes, observe the colour of the liquid in both test tubes
Results and Analysis
- You should notice that at the higher temperature, more of the pigment has leaked out of the beetroot
- This is because:
- The cell membrane of the beetroot cells has become damaged so more pigment can leak out
- At higher temperatures, particles have more kinetic energy, this results in the faster movement of particles compared to when they have less energy


Investigating the effect of temperature on diffusion in beetroot
Limitations
- The beetroot pieces may not be identical in size and shape, meaning one test tube could contain slightly more beetroot tissue than the other
- Solution: cut the beetroot as accurately as possible using a knife and ruler, and repeat each investigation several times to find a mean
- Some parts of beetroot tissue could have more pigment in their cells than others
- Solution: conduct several repeats, using different parts of the beetroot and find a mean
- Our results would be more reliable if we tested a range of temperatures rather than just testing 2
- Solution: Set up 5 test tubes in water baths at different temperatures (e.g. 10℃, 20℃, 30℃, 40℃, 50℃)
- Observing the colour is a subjective measure which means it is difficult to really compare the differences in diffusion between the test tubes
- Solution: use a colorimeter to measure how much light is absorbed as it passes through each of the five samples of coloured liquid
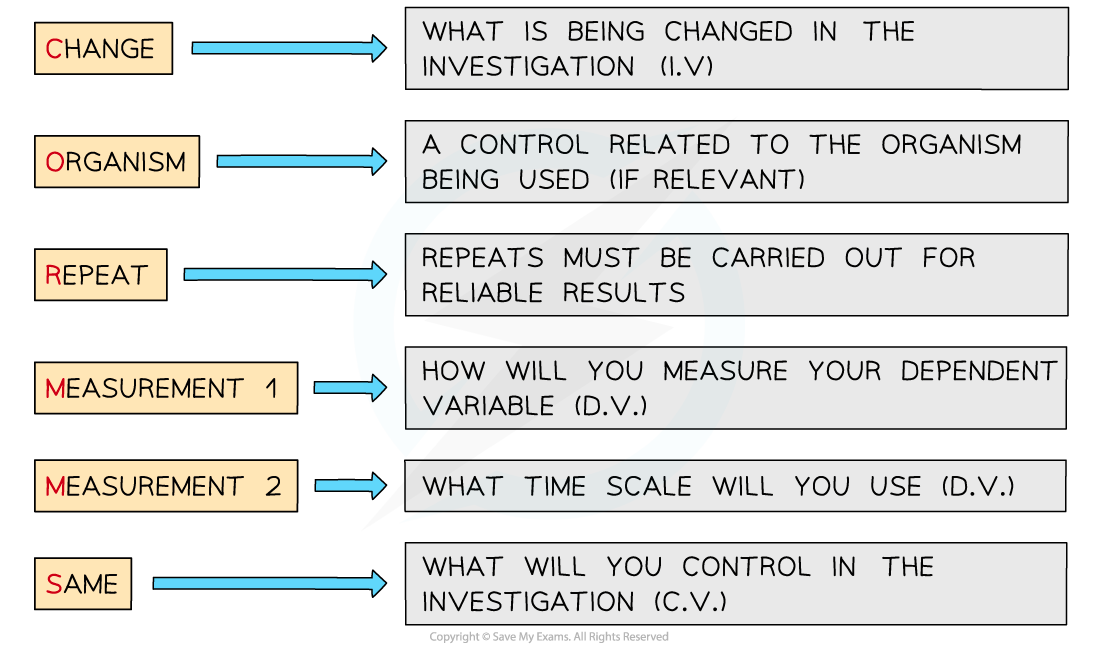
Applying CORMS to practical work
- When working with practical investigations, remember to consider your CORMS evaluation
CORMS evaluation
- In this investigation, your evaluation should look something like this:
- C - We are changing the temperature of the environment
- O - The beetroot cubes will all be taken from the same beetroot or beetroot of the same age
- R - We will repeat the investigation several times to ensure our results are reliable
- M1 - We will observe the colour change of the liquid
- M2 - ...after 10 minutes
- S - We will control the volume of water used, the dimensions of the beetroot cubes and each cube must be blotted before it is weighed each time
Practical: Factors that Influence Osmosis
- Osmosis is the diffusion of water molecules from a dilute solution (high concentration of water) to a more concentrated solution (low concentration of water) across a partially permeable membrane

Osmosis in cells
- We can investigate osmosis using cylinders of potato and placing them into distilled water and sucrose solutions of increasing concentration
Apparatus
- Potatoes
- Cork borer
- Knife
- Sucrose solutions (from 0 Mol/dm3 to 1 mol/dm3)
- Test tubes
- Balance
- Paper towels
- Ruler
- Test tube rack
Method
- Prepare a range of sucrose (sugar) solutions ranging from 0 Mol/dm3 (distilled water) to 1 mol/dm3
- Set up 6 labelled test tubes with 10cm3 of each of the sucrose solutions
- Using the knife, cork borer and ruler, cut 6 equally-sized cylinders of potato
- Blot each one with a paper towel and weigh on the balance
- Put 1 piece into each concentration of sucrose solution
- After 4 hours, remove them, blot with paper towels and reweigh them



Experimental method for investigating osmosis in potato cylinders
Results and analysis
- The percentage change in mass can be calculated for each piece of potato

Calculating percentage change in mass
- The potato cylinder in the distilled water will have increased its mass the most as there is a greater concentration gradient in this tube between the distilled water (high water potential) and the potato cells (lower water potential)
- This means more water molecules will move into the potato cells by osmosis, pushing the cell membrane against the cell wall and so increasing the turgor pressure in the cells which makes them turgid - the potato cylinders will feel hard
- The potato cylinder in the strongest sucrose concentration will have decreased its mass the most as there is a greater concentration gradient in this tube between the potato cells (higher water potential) and the sucrose solution (lower water potential)
- This means more water molecules will move out of the potato cells by osmosis, making them flaccid and decreasing the mass of the cylinder - the potato cylinders will feel floppy
- If looked at underneath the microscope, cells from this potato cylinder might be plasmolysed, meaning the cell membrane has pulled away from the cell wall

Plasmolysed red onion cells
- If there is a potato cylinder that has not increased or decreased in mass, it means there was no overall net movement of water into or out of the potato cells
- This is because the solution that the cylinder was in was the same concentration as the solution found in the cytoplasm of the potato cells, so there was no concentration gradient
Limitations
- Slight differences in potato cylinders may mean that results aren't reliable or comparable
- Solution: for each sucrose concentration, repeat the investigation with several potato cylinders. Making a series of repeat experiments means that any anomalous results can be identified and ignored when a mean is calculated
Applying CORMS evaluation to practical work
- When working with practical investigations, remember to consider your CORMS evaluation

CORMS evaluation
- In this investigation, your evaluation should look something like this:
- C - We are changing the concentration of sucrose solution
- O - The potato cylinders will all be taken from the same potato or potatoes of the same age
- R - We will repeat the investigation several times to ensure our results are reliable
- M1 - We will measure the change in mass of the potato cylinders
- M2 - ...after 4 hours
- S - We will control the volume of sucrose solution used, the dimensions of the potato cylinders and each cylinder must be blotted before it is weighed each time
Exam Tip
Questions involving osmosis experiments are common and you should be able to use your knowledge of these processes to explain the results.Don’t worry if it is an experiment you haven’t done – simply figure out where the higher concentration of water molecules is – this is the solution with the higher water potential - and explain which way the molecules move due to the differences in water potential.

You've read 0 of your 0 free revision notes
Get unlimited access
to absolutely everything:
- Downloadable PDFs
- Unlimited Revision Notes
- Topic Questions
- Past Papers
- Model Answers
- Videos (Maths and Science)
Did this page help you?