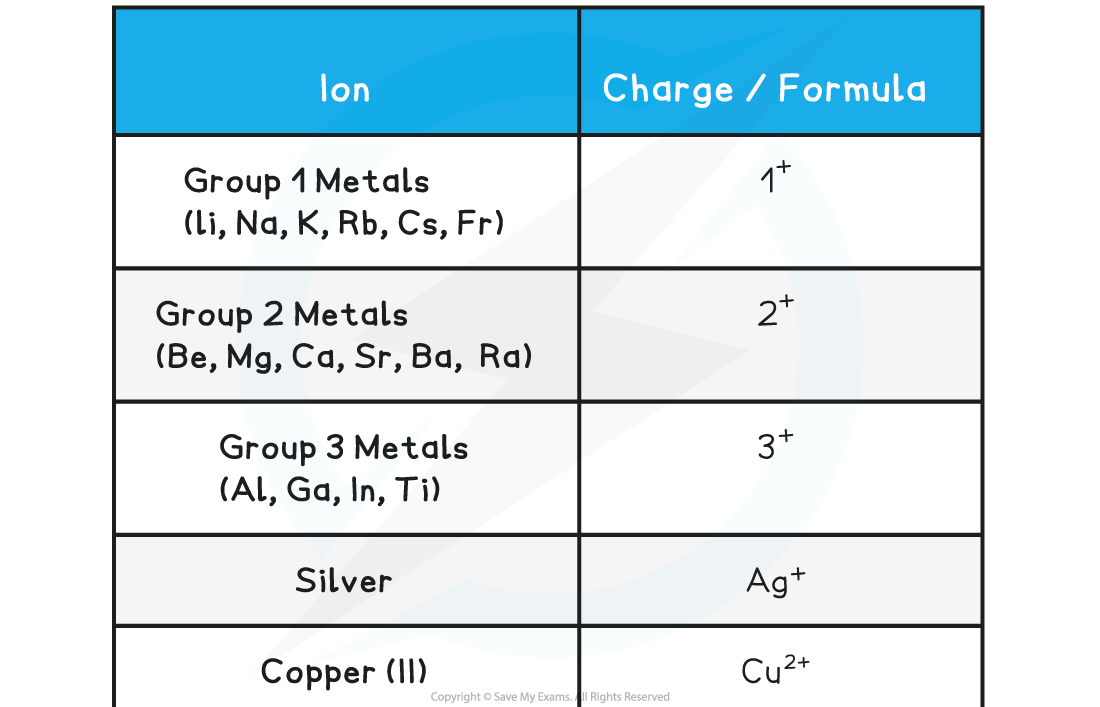
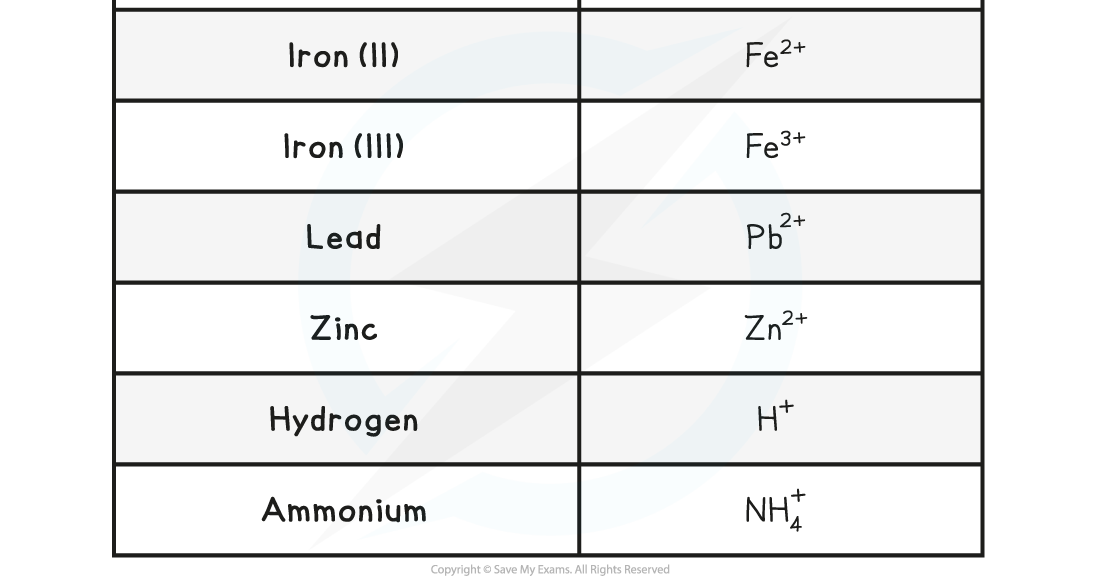
Common Ions
How to deduce the charge of an ion
- Find the number of electrons in the outer electron shell
- Find out if it is easy for the atom to gain electron or to donate electron (in most cases atoms that have fewer than four electrons, donate electrons and atoms that have more than 4 electrons, receive electrons)
- Atoms that gain electrons become negative ions and atoms that donate electron forms positive ion
- You also need to learn the formula of compound ions, that is, ions made from more than one element
The Charges of Common Positive Ions Table
The Charges of Common Negative Ions Table


