Redox Titrations
- Redox titration involves an oxidising agent being titrated against a reducing agent
- Electrons are transferred from one species to another
- In acid-base titrations indicators are used to show the endpoint of a reaction; however redox titrations using transition metal ions naturally change colour when changing oxidation state, so indicators are not always necessary
- They are said to be 'self-indicating'
- Two common transition metal ion redox titrations are manganate(VII) and dichromate(VI) titrations
Manganate(VII) Titrations
- Potassium manganate(VII) is an oxidising agent and is a deep purple colour
- In acidic solutions it is reduced to the almost colourless manganese(II) ion (the ion is actually pink, but in low concentrations it is effectively colourless)
- The reduction equation for the manganate(VII) ion is
MnO4- (aq) + 8H+ (aq) + 5e- → Mn2+ (aq) + 4H2O (aq)
purple colourless
- The potassium ion is a spectator ion so can be left out of the equations
- By convention the potassium manganate(VII) solution is placed in the burette, so that as it reacts with the reducing agent the solution becomes colourless
- At the endpoint the manganate(VII) ion becomes in excess so the first appearance of a permanent colour change marks the endpoint
- The colour seen is pink; if the colour is purple then you have overshot the endpoint and there is too much manganate(VII) in excess
- Analysis of iron in iron(II)sulfate tablets is typical manganate(VII) titration
Worked example
A health supplement tablet contain iron(II)sulfate was analysed by titration. A tablet weighing 2.25 g was dissolved in dilute sulfuric acid and titrated against 0.100 mol dm-3 KMnO4 .The titration required 26.50 cm3 for complete reaction. Calculate the percentage by mass of iron in the table.
Answer
Step 1: Write the balanced equation for the reaction
oxidation: Fe2+ (aq) → Fe3+ (aq) + e-
reduction: MnO4- (aq) + 8H+ (aq) + 5e- → Mn2+ (aq) + 4H2O (l)
overall: MnO4- (aq) + 8H+ (aq) + 5Fe2+ (aq) → Mn2+ (aq) + 4H2O (l) + 5Fe3+ (aq)
Step 2: Determine the amount of MnO4- used in the titration
moles of MnO4- = 0.0265 dm3 x 0.100 mol dm-3 = 0.00265 mol
Step 3: Determine the amount of iron in the reaction
From the equation for the reaction we know the reacting ratio MnO4- : Fe2+ = 1: 5
∴ moles of Fe2+ = 0.00265 mol MnO4- x 5 = 0.01325 mol
Step 4: Convert moles into mass of iron
Mass of iron = 0.01325 mol x 55.85 gmol-1 = 0.740 g
Step 5: find the percentage of iron in the tablet
∴ % Fe in the tablet = (0.740/ 2.25) x 100 = 32.9%
Dichromate(VI) Titrations
- Potassium dichromate(VI), K2Cr2O7, is another oxidising agent used in redox titrations
- The oxidation state of the chromium changes from +6 to +3 in the reaction:
Cr2O7- (aq) + 14H+ (aq) + 6e- → 2Cr3+ (aq) + 7H2O (l)
orange green
- From the half equation you can see that the reaction needs hydrogen ions so the solution must be acidified with excess dilute sulfuric acid
- The colour change is hard to see as the dichromate(VI) ion is pale orange and the chromium(III) ion is pale green
- To enhance the endpoint an indicator called diphenylaminesulfonate is used which turns from colourless to purple at the endpoint
- To obtain the balanced equation in the analysis of iron(II) first work out the redox changes in the half equations, then balance the number of electrons transferred:

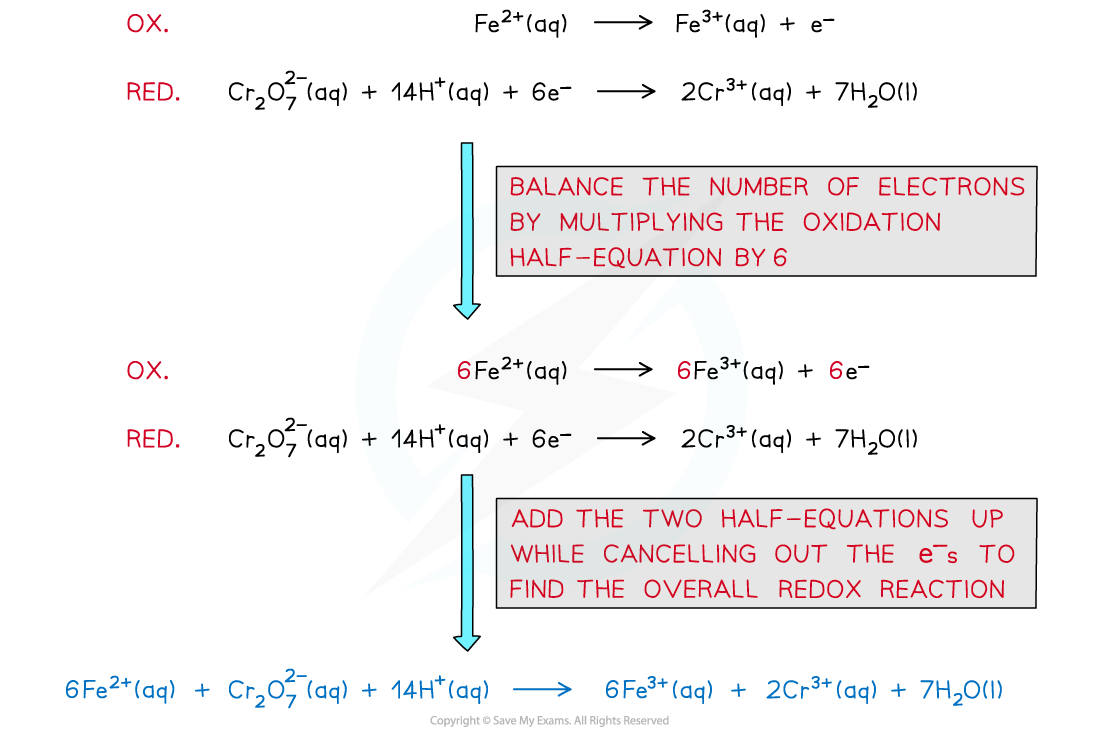
The steps in producing a balanced redox equation between iron(II) and dichromate(VI)
Exam Tip
Always show your working in redox titration problems as marks can be awarded for the steps even if the final answer is wrong.

