Reactivity Trend
Group 1 metals
- The reactivity of the group 1 metals increases as you go down the group
- When a group 1 element reacts its atoms only need to lose electron, as there is only 1 electron in the outer shell
- When this happens, 1+ ions are formed
- The next shell down automatically becomes the outermost shell and since it is already full, a group 1 ion obtains noble gas configuration
- As you go down group 1, the number of shells of electrons increases by 1
- This means that the outermost electron gets further away from the nucleus, so there are weaker forces of attraction between the outermost electron and the nucleus
- Less energy is required to overcome the force of attraction as it gets weaker, so the outer electron is lost more easily
- So, the alkali metals get more reactive as you descend the group
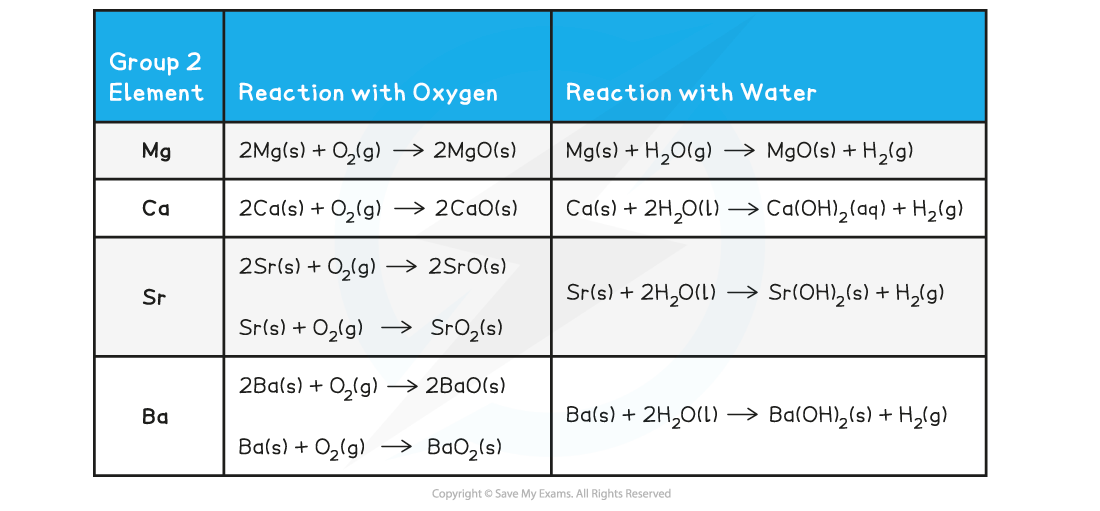
Group 2 metals
- The reactivity of the Group 2 metals also increases down the group for the same reasons
- The outermost electron gets further away from the nucleus, so there are weaker forces of attraction between the outermost electron and the nucleus
- Less energy is required to overcome the force of attraction as it gets weaker, so the outer electron is lost more easily
- This can be observed when the Group 2 metals react with water as well
- Magnesium reacts extremely slowly with cold water
- Calcium reacts fairly vigorously with cold water in an exothermic reaction