Enzymes are Catalysts
- Within an organism there are thousands of chemical reactions happening within the cells
- e.g. the process of photosynthesis employs a host of chemical reactions to generate a useful end-product which is glucose
- Metabolism is the sum of all the reactions happening in a cell or organism, in which molecules are synthesised (made) or broken down
- Chemical reactions need to be carefully controlled to ensure the right amount of a particular substance is made
- It is usually beneficial for the cell if chemical reactions happen quickly
- raising the temperature can speed up chemical reactions
- but this would speed up any unwanted reactions too
- If the internal temperature is raised too much the cells will become damaged
Enzymes help control cell reactions
- Enzymes act as biological catalysts to speed up the rate of a chemical reaction without being changed or used up in the reaction
- Enzymes reduce the need for high temperatures
- They are biological as they are made in cells
- Enzymes are necessary to all living organisms as they allow all metabolic reactions to occur at a rate that can sustain life
- For example, if we did not produce digestive enzymes, it would take around 2 - 3 weeks to digest one meal; with enzymes, it takes around 4 hours
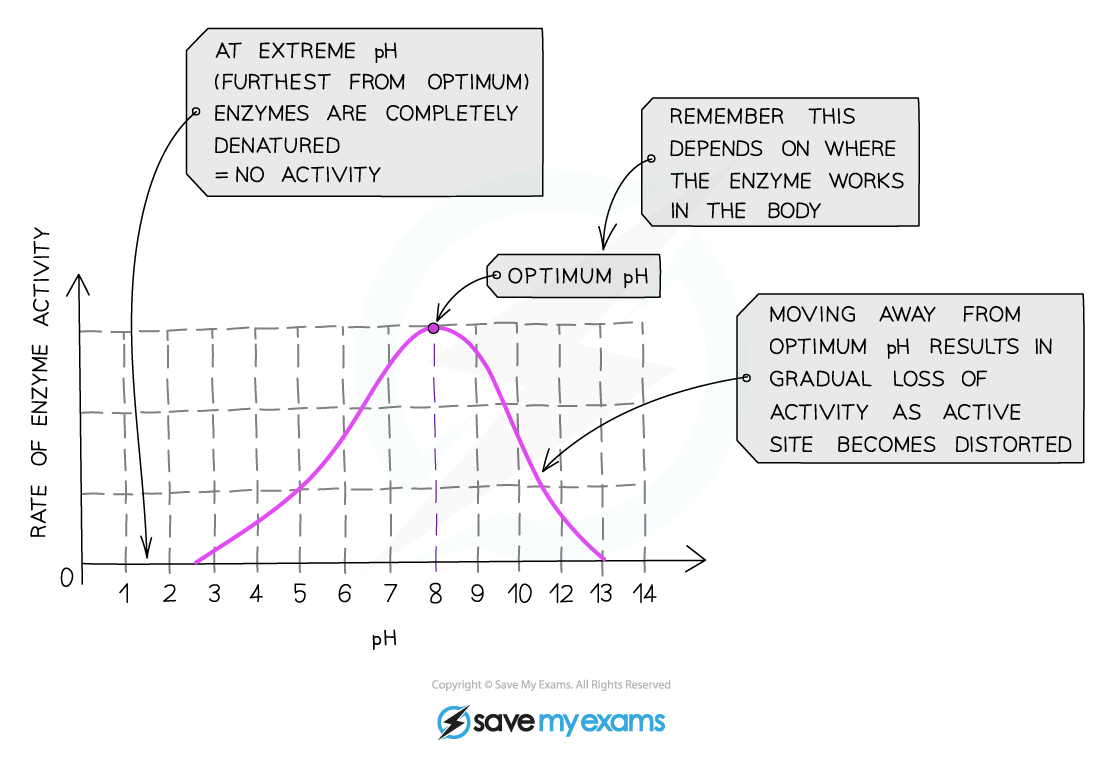
- Different biological reactions all have a specific enzyme to help control the reaction
- Enzymes are made of protein and have a unique shape to help them function
The active site
- Chemical reactions usually involve changing a particular molecule
- which can be split apart or joined to another molecule
- The molecule being changed is known as the substrate
- Each enzyme has a specially shaped region known as an active site
- The active site allows the enzyme to bind to the substrate
- Once bound to the active site, the chemical reaction takes place
- Enzymes usually only work with one type of substrate - this is known as specificity
- The specificity of an enzyme is a result of the complementary nature between the shape of the active site on the enzyme and its substrate(s)

Enzymes are biological catalysts that work in cells, so they randomly move about wherever they are in the cell. They don’t ‘choose’ to collide with a substrate – collisions occur because all molecules are in motion in a liquid