Electronic Structure (AQA GCSE Chemistry)
Revision Note

Author
StewartExpertise
Chemistry Lead
Electron Shell Diagrams
- We can represent the electronic structure of atoms using electron shell diagrams
- Electrons orbit the nucleus in shells
- Each shell has a different amount of energy associated with it
- The further away from the nucleus, the more energy a shell has
- Electrons fill the shell closest to the nucleus
- When a shell becomes full of electrons, additional electrons have to be added to the next shell
- The first shell can hold 2 electrons
- The second shell can hold 8 electrons
- For GCSE, a simplified model is used that suggests that the third shell can hold 8 electrons
- For the first 20 elements, once the third shell has 8 electrons, the fourth shell begins to fill
- The outermost shell of an atom is called the valence shell and an atom is much more stable if it can manage to completely fill this shell with electrons
- In most atoms, the outermost shell is not full and therefore these atoms react with other atoms in order to achieve a full outer shell of electrons (which would make them more stable)
- In some cases, atoms lose electrons to entirely empty this shell so that the next shell below becomes a (full) outer shell
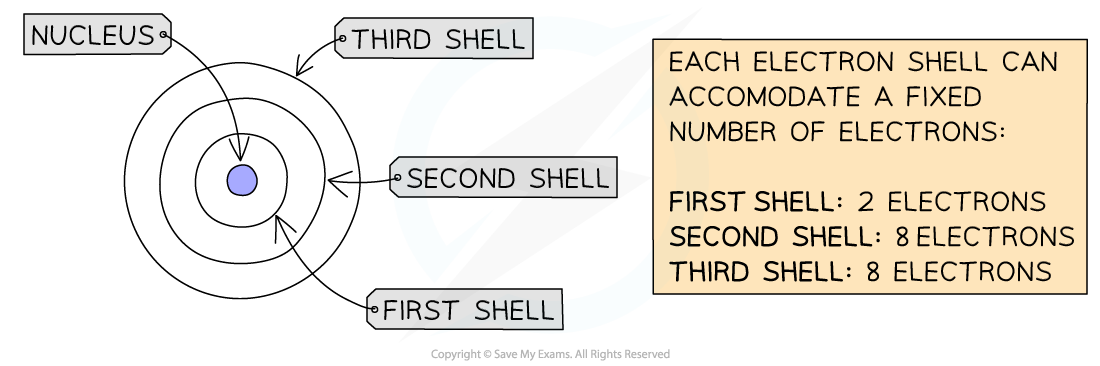
The electron shells
Exam Tip
All of the shells up to the outer shell will be full. Electron transfer occurs with electrons from the outer shell only. You can use the term ‘shell’, 'orbital' or ‘energy level’ to describe the space that electrons occupy.
Electronic Configuration: Number Notation
- The arrangement of electrons in shells can also be explained using numbers
- This method consists of writing down the number of electrons in each shell and separating each number with a comma
- There is a clear relationship between the outer shell electrons and how the periodic table is designed
- The number of notations in the electronic configuration will show the number of shells of electrons the atom has, showing the period in which that element is in
- The last notation shows the number of outer electrons the atom has, showing the group that element is in
- Elements in the same group have the same number of outer shell electrons

The electronic configuration for chlorine
- Period: The red numbers at the bottom show the number of notations which is 3, showing that a chlorine atom has 3 shells of electrons
- Group: The last notation, in this case 7, shows that chlorine has 7 outer electrons, and therefore is in group 7

The position of chlorine on the Periodic Table
Electronic Configuration: First 20 Elements
Electronic Configuration of the First 20 Elements Table
Note: The GCSE model for electronic configuration is a simplified model. The third shell of electrons can actually hold up to 18 electrons. But filling these shells follows a more complicated pattern due to energy levels and stability. This is why potassium and calcium fill the third shell with 8 electrons and then put their final electrons in the fourth shell.
Exam Tip
You should be able to represent the first 20 elements using either electron shell diagrams or written electronic configuration.

You've read 0 of your 0 free revision notes
Get unlimited access
to absolutely everything:
- Downloadable PDFs
- Unlimited Revision Notes
- Topic Questions
- Past Papers
- Model Answers
- Videos (Maths and Science)
Did this page help you?