Write one equation to represent each the following changes:
Atomisation of sodium ………………………………………………………………….
First ionisation energy of magnesium ………………………………………………………………….
First electron affinity of chlorine ………………………………………………………………….
Give the definition of the term enthalpy of lattice formation.
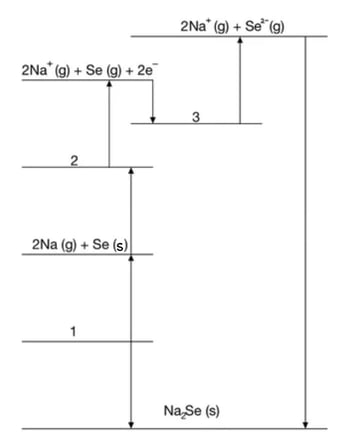
Study the following Born-Haber cycle.

State the enthalpy changes for the following steps:
Step 1 ………………………………………………………………………………………………..
Step 3 ………………………………………………………………………………………………..
Step 4 ………………………………………………………………………………………………..
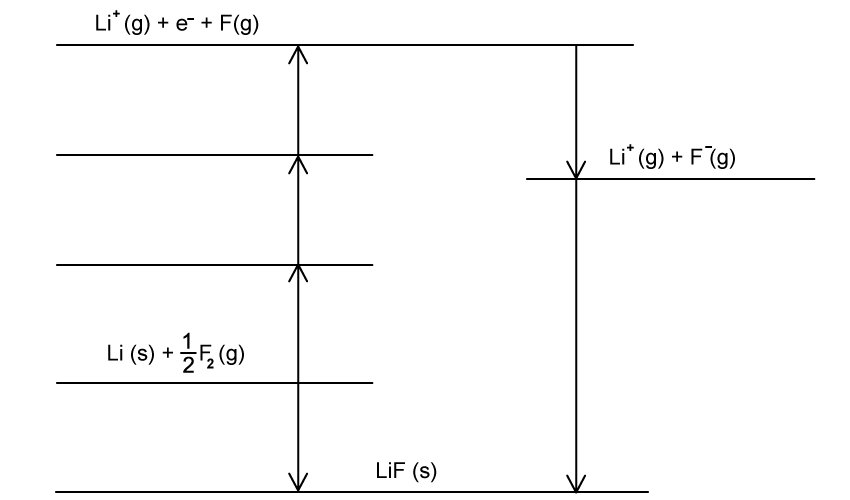
The enthalpy of lattice formation of potassium fluoride and caesium fluoride is -829 kJ mol-1 and -759 kJ mol-1 respectively.
With reference to the ions in the structure, explain why the enthalpy of lattice formation is more exothermic for potassium fluoride.
Did this page help you?